A new paradigm in acne vulgaris
Maurice A.M. van Steensel M.D., Ph.D.
April 5, 2023
Introduction
In this article, I will outline the current understanding of acne vulgaris, the most common skin disease. It departs from the old paradigm, which assigns a major role to sebum and the bacterium Cutibacterium acnes. We have shown that acne is not primarily an in ammatory disorder that has nothing to do with either sebum or bacteria. Instead, it is best seen as a condition that is brought about by abnormal di erentiation of sebaceous progenitor cells. This is what causes comedones to form. Everything else is secondary. This new conceptual framework can explain observations that the old one could not. It also provides a mechanism of action for retinoids and indicates novel therapeutic targets.
Shifting perspectives: from bacteria to stem cells
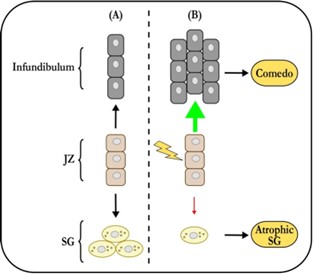
The classic paradigm of acne pathogenesis proposes that, at the onset of puberty, increased sebum production by enlarging sebaceous glands (SG) facilitates the growth of pathogenic C. acnes strains [6]. This might be the consequence of altered sebum composition, which has certainly been reported in acne. The bacteria then cause comedones and/or in ammation, although comedogenesis is commonly ascribed to blockage of the infundibulum. This sequence of events has for many years been accepted as the truth about acne, even if it doesn’t really explain anything. Many questions remain, which I have previously reviewed in [10]. For example, why would the SG associated with a comedo be atrophic if excess sebum is the root cause of acne? If altered sebum composition is the culprit, then why is such a small proportion of pilosebaceous units actually a ected? Why would sebum composition be abnormal in the rst place? How can acne be purely comedonic? Et cetera, et cetera.
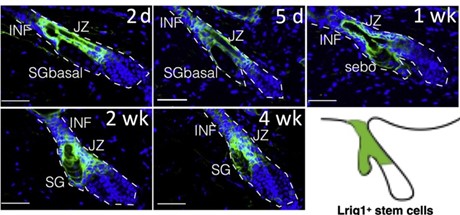
Clearly, there is a lack of explanatory power here. To arrive at a deeper un- derstanding, we must revisit two well established observations. First, acne is not an SG disease – comedones are lesions of the junctional zone (JZ), where the se- baceous duct joins the infundibulum. Second, sebaceous glands associated with comedones are always atrophic. So, what does this mean? Well, the JZ houses the progenitor cells that support the sebaceous gland’s growth and maintenance ( gure 1). Solid experimental evidence indicates that these cells can contribute either to the infundibulum, or to the sebaceous gland. The latter contribution needs to increase massively at the onset of puberty, when the sebaceous gland grows to its adult size. The progenitors are also required for maintenance of the holocrine SG. If its basal cell layer is not regularly replenished, it will disap- pear. So, if the SG progenitors can assume one of two identities, there must be a switch to tell them which one to pick. There is one, and Saurat was the rst (in 2015) to suggest that its failure initiates comedogenesis by favoring infundibular over SG identity [8]. It turns out that he was right and there is a switch, which is operated by Wingless signaling. Anything that disrupts it can cause come- dones to form. It is worth noting that this simple comedo switch hypothesis explains many features of acne that the old paradigm either has trouble with or cannot. For example, it provides a clear mechanism for the SG atrophy that accompanies comedogenesis.
How it works
It is well established that sebaceous progenitor cells in the JZ express the marker Lrig1, in mice as well as in humans [1]. Using Lrig1 to target a gene knockout, we modulated Wingless (Wnt) signaling in mouse sebaceous progenitors, testing the hypothesis that Wnt controls their di erentiation. Wingless signaling is a highly conserved system that controls stem cell di erentiation in many organs, including the skin [2]. As shown in gure 2, activating Wnt signaling in se- baceous progenitors by knocking out the Wnt suppressor gene Apc results in rapid, comedo-like expansion of the JZ. Of note, this phenomenon is associated with profound atrophy of the SG. Just like in acne. But you will say, surely this is not what happens in actual patients with acne? Indeed, it isn’t, as the average patient with acne does not have an Apc knockout. However, recent genome-wide association studies (GWAS) show that our mouse model is not far from the human situation. GWAS unequivocally implicate Wingless in the pathogenesis of sporadic acne [7].
In a striking parallel with our mouse data, gain-of-function alleles in WNT10A (leading to increased Wingless signaling) are the single strongest genetic risk fac- tor for developing the condition [7]. Accordingly, loss-of-function alleles were found to be protective. These ndings are consistent across ethnic groups in multiple studies. GWAS also highlight signals related to tissue remodeling in addition to cell proliferation and migration. Monogenic disorders that cause severe acne a ect the same processes [4]. This makes sense if one realises that sebaceous progenitor cells, once switched to a sebaceous fate, need to move into the sebaceous gland and di erentiate at the same time. If the movement is impeded (tissue remodeling defect), or the mechanisms that facilitate it over- whelmed (hyperandrogenism), the progenitors will get stuck in the JZ causing it to expand. A comedo will result – in essence, a cystic expansion of the abnor- mally di erentiated sebaceous progenitor compartment.
What it means
The above scenario requires no micro-organisms to work. Only androgens, ge- netic predisposition and environmental factors such as pollutants. It explains chloracne, where it was shown that dioxins disrupt the sebaceous di erentiation of Lrig1+ progenitor cells [5]. Importantly, it o ers a straightforward mecha- nism for the strong e ects of retinoids on acne, including their ability to prevent disease recurrence. In our Apc conditional knockout mouse, topical application of ATRA prevented formation of the comedo-like cysts by inhibiting Wnt sig- naling. We further showed in our mouse models that Wnt inhibition causes sebaceous glands to enlarge, with widening, but not cystic expansion, of the in- fundibulum. These observations suggest that the therapeutic e ects of retinoids on acne are due to promotion of sebaceous di erentiation and, probably, inhi- bition of sebaceous progenitor growth. We have observed that 13-cis retinoic acid (Accutane) keeps human sebaceous progenitor cells from forming organoids (unpublished data). In conclusion, retinoids probably cure acne by inhibiting Wnt signaling, thence promoting sebaceous di erentiation and inhibiting pro- genitor proliferation. Note that this mechanism explains how retinoids induce sebaceous gland atrophy and, thence, dry skin.
Conclusion
Until very recently, we had no in vitro models for the key events in the patho- genesis of acne. But now we have human sebaceous organoids, formed from progenitor cells. Using these, we can now model early events in sebaceous di erentiation and observe how those are a ected by genetics, drugs or environ- mental factors such as pollutants. In this way, we will be able to understand the mechanism by which these agents can cause acne and come up with preventive or therapeutic interventions. Identifying these is now possible, as organoids can be grown at scale and hence can be used for high-throughput screening. We expect that people with acne will stand to bene t soon from our new insights and capabilities.
Acknowledgments
Work on acne in my laboratory is made possible by generous funding from Lee Kong Chian School of Medicine (startup grant) and the National Med- ical Research Councel, Clinician-Scientist Individual Research Grant MOH- CIRG20nov-0009.
References
- Laurent Barnes, Joeri Puenchera, Jean-Hilaire Saurat, and G rkan Kaya. Lrig1 and cd44v3 expression in the human folliculosebaceous unit. Dermatology (Basel, Switzerland), 231:116 118, 2015.
- Andy J. Chien, William H. Conrad, and Randall T. Moon. A wnt survival guide: from ies to human disease. The Journal of investigative dermatol- ogy, 129:1614 1627, July 2009.
- R. W. Clayton, K. G bel, C. M. Niessen, R. Paus, M. A. M. van Steensel, and X. Lim. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. The British journal of dermatology, 181:677 690, October 2019.
- J E A Common, J N Barker, and M A M van Steensel. What does acne ge- netics teach us about disease pathogenesis? Br J Dermatol, 181(4):665 676, October 2019.
- Fabienne Fontao, Laurent Barnes, Guerkan Kaya, Jean-Hilaire Saurat, and Olivier Sorg. From the cover: High susceptibility of lrig1 sebaceous stem cells to tcdd in mice. Toxicological sciences : an o cial journal of the Society of Toxicology, 160:230 243, December 2017.
- Noreen Mohsin, Loren E. Hernandez, Mackenzie R. Martin, Ashley Vander Does, and Keyvan Nouri. Acne treatment review and future perspectives. Dermatologic therapy, 35:e15719, September 2022.
- Christos Petridis, Alexander A. Navarini, Nick Dand, Jake Saklatvala, David Baudry, Michael Duckworth, Michael H. Allen, Charles J. Curtis, Sang Hyuck Lee, A. David Burden, Alison Layton, Veronique Bataille, Andrew E. Pink, Acne Genetic Study Group, Isabelle Carlavan, Jo- hannes J. Voegel, Timothy D. Spector, Richard C. Trembath, John A. McGrath, Catherine H. Smith, Jonathan N. Barker, and Michael A. Simp- son. Genome-wide meta-analysis implicates mediators of hair follicle devel- opment and morphogenesis in risk for severe acne. Nature communications, 9:5075, December 2018.
- Jean-Hilaire Saurat. Strategic targets in acne: The comedone switch in question. Dermatology (Basel, Switzerland), 231:105 111, 2015.
- Wei Shang, Alvin Yong Quan Tan, Maurice A M van Steensel, and Xinhong Lim. Aberrant Wnt Signaling Induces Comedo-Like Changes in the Murine Upper Hair Follicle. J Invest Dermatol, 142(10):2603 2612.e6, October 2022.
- Maurice A M van Steensel and Boon Chong Goh. Cutibacterium acnes: Much ado about maybe nothing much. Exp Dermatol, 30(10):1471 1476, October 2021.














